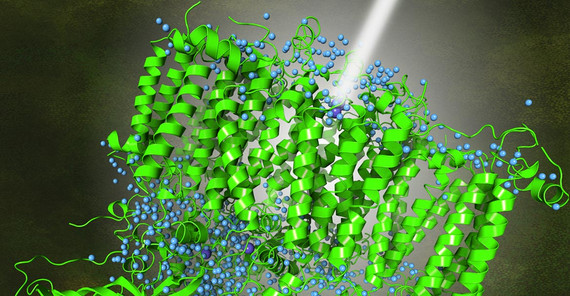
Researchers from Humboldt University Berlin, Umeå University, Uppsala University, and the University of Potsdam have achieved a significant breakthrough in understanding the intricate processes of photosynthesis. Their study leverages high-resolution cryo-electron microscopy (cryo-EM) to uncover detailed structures of Photosystem II (PS II), which is one of two photosystems of organisms that release oxygen during photosynthesis. PS II is stimulated by solar radiation. The light energy causes the splitting of a water molecule, which leads to the formation of electrons, protons and oxygen.
The research team has successfully mapped the PS II structure at an unprecedented resolution of 1.7 Ångström (1.7x10-10 meters). This allows the positions of the hydrogen atoms and protons in PS II to be precisely determined. This detailed view is crucial for understanding how oxygenic photosynthetic organisms convert light energy into chemical energy – a process that is fundamental to life on Earth.
The cryo-EM technique has a broad spectrum of applications and can be applied to study various molecules, which is relevant in multiple areas of biological and chemical research. “Cryo-EM is evolving into a versatile technique that addresses challenges extending beyond the mere elucidation of protein complex structures,” says Prof. Dr. Petra Wendler from the University of Potsdam, who contributed to the cryo-EM data analysis.
Original Publication: Rana Hussein, André Graça, Jack Forsman, A. Orkun Aydin, Michael Hall, Julia Gaetcke, Petko Chernev, Petra Wendler, Holger Dobbek, Johannes Messinger, Athina Zouni, Wolfgang P. Schröder, Cryo–electron microscopy reveals hydrogen positions and water networks in photosystem II. Science384, 1349-1355 (2024). www.science.org/doi/10.1126/science.adn6541
Link to press release of Humboldt-Universität zu Berlin: https://www.hu-berlin.de/en/press-portal/nachrichten-en/june-2024/nr-24621
Contact:
Prof. Dr. Petra Wendler
Institute of Biochemistry and Biology
Tel.: 0331 977-5130
E-Mail: petra.wendleruuni-potsdampde