Research
Site-specific conjugate synthesis
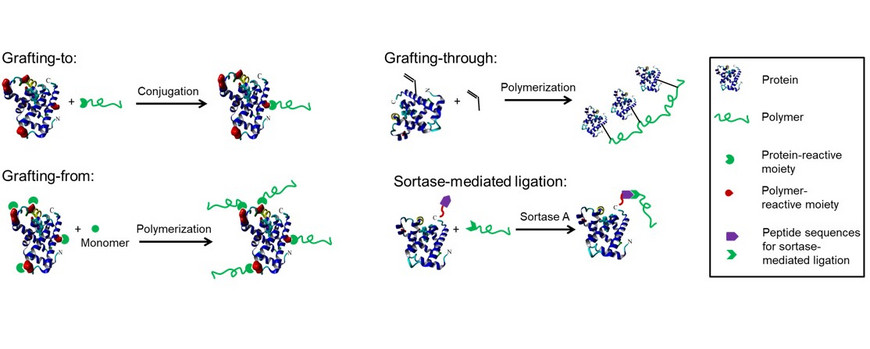
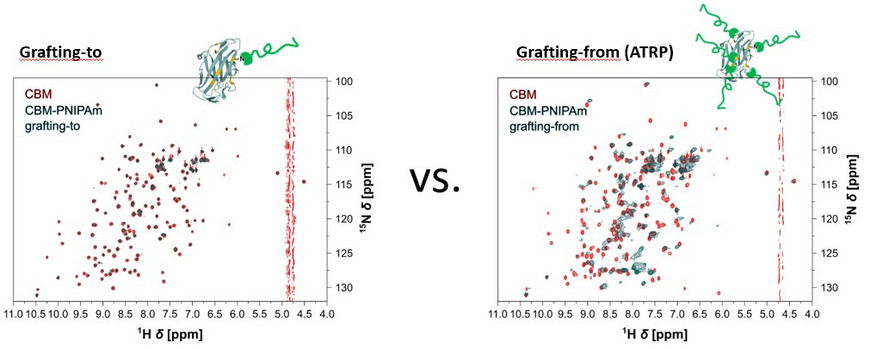
Two approaches are typically used for the synthesis of protein-polymer conjugates, grafting-to and grafting-from. During grafting-to, the polymer is synthesized first and subsequently conjugated to the protein. For grafting-from, a polymerization initiator or mediator is linked to the protein followed by grafting the polymers directly from the protein surface. The third, seldom used technique, grafting-through, consists of the polymerization of peptide or protein monomers to incorporate several biomolecules in the polymer backbone. Grafting-to and grafting-from suffer from low specificity, especially when targeting the abundant lysine residues. Enzymatic strategies are a promising approach for a site-specific polymer attachment. We are currently using sortase-mediated ligation (SML) for C- or N-terminal modification of proteins.
Macromol. Biosci., 2025, 25, 2400316
RSC Adv., 2019, 9, 4700
Polym. Chem., 2015, 6, 5143
Stabilization of enzymes
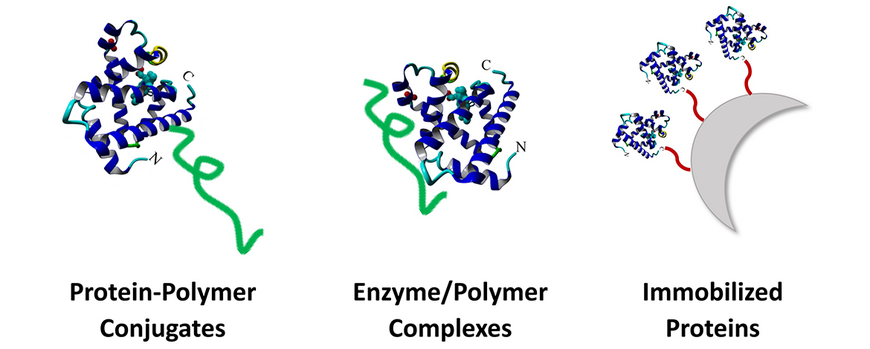
Enzymes are very attractive alternatives to conventional catalysts due to their high specificity, versatility and adaptability. However, enzymes are sensitive biomolecules that can be quickly inactivated by unnatural conditions such as high temperatures and high or low pH values. Our goal is to stabilize enzymes using synthetic polymers so that they can be used at higher temperatures and over longer periods of time without losing their activity. Our approaches include the covalent conjugation of polymers to enzymes, the formation of non-covalent enzyme/polymer complexes, and the immobilization of the biocatalysts on polymeric carrier materials. While polymer conjugation and complexation can improve stability and activity, immobilization also allows the separation and reuse of enzymes.
Cooperation partners:
Prof. Dr. Leilei Zhu, Tianjin Institute of Industrial Biotechnology TIB, China
Dr. Antje Lieske, Fraunhofer Institute for Applied Polymer Research IAP, Potsdam
Dr. Mehdi D. Davari, Leibniz Institute for Plant Biochemistry IPB, Halle
Prof. Dr. Katja Arndt, University of Potsdam
Dr. Martin Reifarth, University of Potsdam
ChemCatChem, 2024, 16, e202301685
Angew. Chem. Int. Ed., 2018, 57, 13810
Therapeutic protein-polymer conjugates
The high specificity of proteins may be advantageous for therapeutic applications compared to small molecule drugs. However, therapeutic proteins are often recognized by the immune system and quickly cleared from the body. It was found in the 1970s that the conjugation of poly(ethylene glycol) (PEG), now known as PEGylation, can shield a protein. As a result, the immunogenicity is reduced and the protein less prone to thermal denaturation, aggregation and degradation by proteases. However, many of the around 20 clinically approved protein-PEG conjugates come along with reduced efficacy, mainly caused by polymer conjugation to random sites in the protein. Furthermore, the development of anti-PEG antibodies, leading to quick clearance of PEGylated drugs, demonstrates that other polymers should be considered for future therapeutic conjugates. We take advantage of our portfolio of protein-polymer conjugation strategies to better understand how to optimize the stabilization of therapeutic proteins. On the one side, we compare different conjugation approaches in their effect on protein structures and functions. On the other side, we synthesize various polymers that are considered as alternatives to PEG and apply them in therapeutic conjugates.
Cooperation partner:
Prof. Dr. Heiko Möller, University of Potsdam
Prof. Dr. Katja Arndt, University of Potsdam
Macromol. Chem. Phys., 2023, 224, 2200353
J. Drug Deliv. Sci. Technol., 2023, 79, 103995
Conjugates for virus inhibition
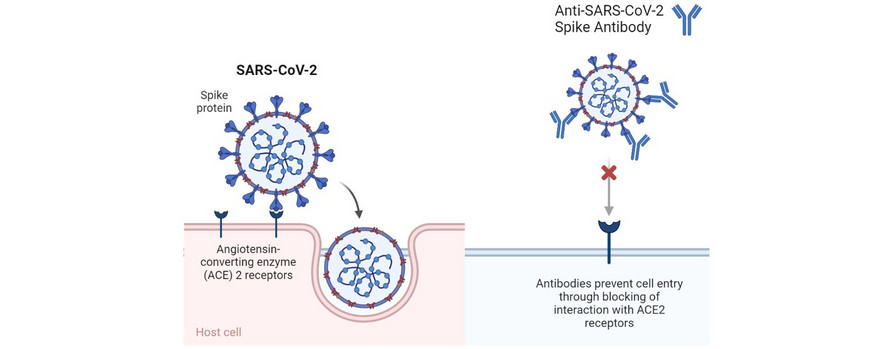
With the emergence of COVID-19, viral infections came into the focus of society, applied and basic research. To date, there are hardly any drugs against viral diseases. SARS-CoV-2 enters human cells after binding of its trimeric spike protein to ACE 2 receptors. Antibodies that bind to surface proteins of viruses and block their interaction with ACE 2 receptors are a therapeutic approach. As the production of antibodies is complex and expensive, nanobodies, small fragments of antibodies, are seen as a potent alternative in basic research. The nanobody Ty1, obtained from alpacas, showed a high potential to neutralize SARS-CoV-2. We synthesize Ty1-polymer conjugates and investigate their stability and potential for virus neutralization. This showed a clear influence of the polymer used, which could open up a wide range of therapeutic options against viral diseases in the future.
Cooperation partner:
Prof. Dr. Petra Wendler, University of Potsdam
Peptide-polymer conjugates to bind lanthanide ions
Lanthanides are of enormous economic importance for many areas of the high-tech industry. They are currently difficult to extract and separate using environmentally hazardous chemicals. As a new approach from the bioeconomy, we are using highly selective peptides with the long-term goal of contributing to sustainability and circular economy. We are currently investigating the extent to which peptide/lanthanide complexation is influenced by polymer conjugation and immobilization.
Cooperation partners:
Prof. Dr. Michael Kumke, University of Potsdam
Prof. Dr. Heiko Möller, University of Potsdam
Dr. Björn Drobot, Helmholtz Center Dresden-Rossendorf
Dr. Franziska Lederer, Helmholtz Center Dresden-Rossendorf
RSC Adv., 2024, 14, 14091
Funding and Acknowlegements
Fraunhofer / UP: high-performance center "Integration of Biological and Physical-Chemical Material Functions", cooperation projects Mat4Water: material platform for the water industry
DFG: "Biohybrid Materials", Heisenberg grant, 02/2024 - 01/2027, project number 530253155
BMBF: “Enzyme Stabilization for a Circular (Bio-)Plastic Economy” (EnzCircEco), 01/2024 - 12/2026, FKZ 031B1424
BMBF: “The development and implementation of a COVId-19 scFv-TRAP portfolio for diagnostic and therapeutic applications” (COVITRAP), 11/2021 – 10/2023, FKZ 01DP21012
BMBF: “Let biology do the trick - Peptides enwind crucial raw materials: the “natural separation” of Lanthanides” (PepTight), 09/2021 – 05/2025, FKZ 031B1122B
Fraunhofer Leistungszentrum "Funktionsintegration": “Sortase-based protein-polymer and protein-nanoparticle conjugates: characterization of 3D-structure, dynamics and Interaction via NMR spectroscopy”, since 01/2020
DFG: “Enabling multiple linkages through sortase-mediated ligation”, 01/2019 – 03/2022, project number 414977640
BMBF: “Chiral Membranes II”, 02/2018 – 01/2021, FKZ 031B0559B