Endocardium biomechanics
Intra-organ communication guides morphogenetic processes that are essential for an organ to carry out complex physiological functions. In the heart, the growth of the myocardium is tightly coupled to that of the endocardium. Yet, was unknown how the growth of these two tissue layers is coordinated. During the process of cardiac ballooning at 30-54 hours post fertilization, endocardial chambers grow by proliferation without accretion of cells from external sources. In striking contrast, the myocardium grows mostly through an accretion of cells to the chamber poles and due to cell size increases. In this study, we used two different genetic conditions to model atrial myocardial chamber expansion and to study the capability of the endocardium to adapt to this enhanced myocardial growth. We found that an increased expansion of the myocardial atrial chamber volume was compensated by increased endocardial proliferation and cell numbers. The mechanism by which this is mediated involves the occurrence of higher junctional forces within endocardial cells. This leads to biomechanical signaling involving VE-cadherin, triggering nuclear localization of the Hippo pathway transcriptional regulator Yap1 and endocardial proliferation. Our work suggests that the growth of the endocardium results from myocardial chamber volume expansion and ends when the tension on the tissue is relaxed (Bornhorst et al., Nature Communications 2019).
The activation of proliferation within the developing endocardium in response to changes in chamber dimensions has important implications not only for our understanding of heart morphogenesis during development but also for characterizing patho-physiological conditions of the heart. It will be of great interest to revisit endocardial chamber development under conditions of congenital heart defects. The present work provides a further component in this repertoire of intra-organ communication which causes biomechanical signaling and tensile force-triggered proliferation within the endocardium. This pattern may also hint at a general blueprint for the coordination of growth rates in the tissues of other complex organs; it shows that homeostatic responses which arise through the morphological adaptations in size of one tissue likely coordinate tissue-intrinsic growth rates within other tissues as well.
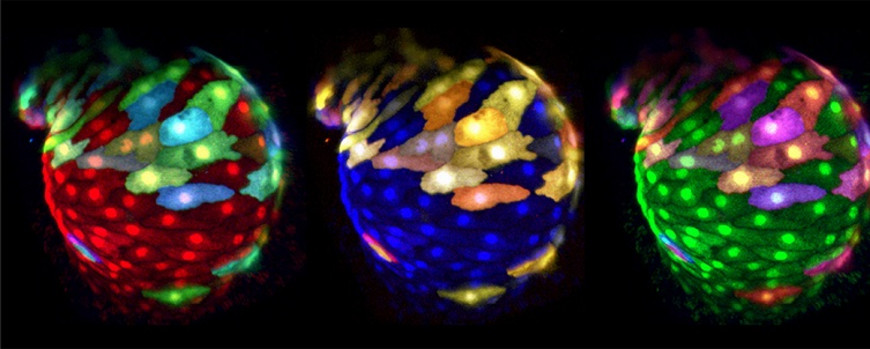
Figure 3: Panel of false-colored reconstructions of multi-color mosaically-labeled zebrafish heart at 50 hpf. Images are taken from a time lapse movie (48-52 hpf). Double transgenic Tg(myl7:EGFP)twu34;Tg(hsp70:wnt8a-GFP)w34 zebrafish were injected with myl7:RFP-T and myl7:BFP Plasmids.