Research
Between spectroscopy, biology, and inorganic chemistry
Our core expertise is Fourier-transformed infrared (FTIR) difference spectroscopy, which we use to investigate biological macromolecules under physiologically relevant, complex conditions. This requires a “trigger” that brings the system out of equilibrium, as functional FTIR spectroscopy is performed “in difference” and allows the smallest changes to be visualized by means of subtraction.
Based on the attenuated total reflectance (ATR) configuration, we have developed different in situ methods for such experiments triggering the reactivity of biological samples and track spectral changes with a temporal resolution of milliseconds to days. As trigger methods, we use gas titrations, potential jump experiments, and photochemistry. In the future we plan to combine ATR FTIR spectroscopy with Raman and UV/Vis spectroscopy. Methods of fast FTIR spectroscopy (µs - ms) are currently under development.
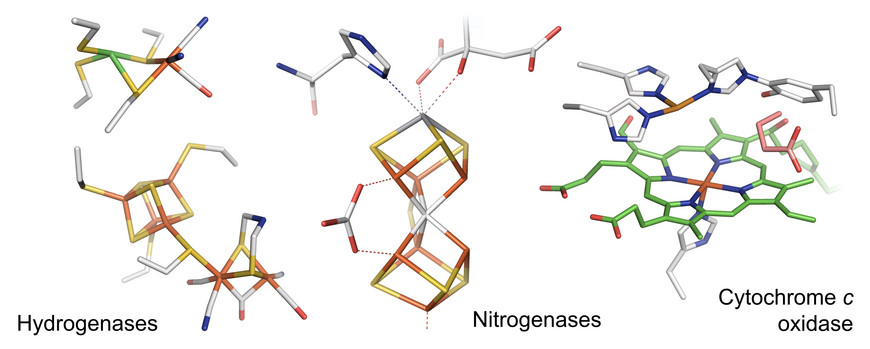
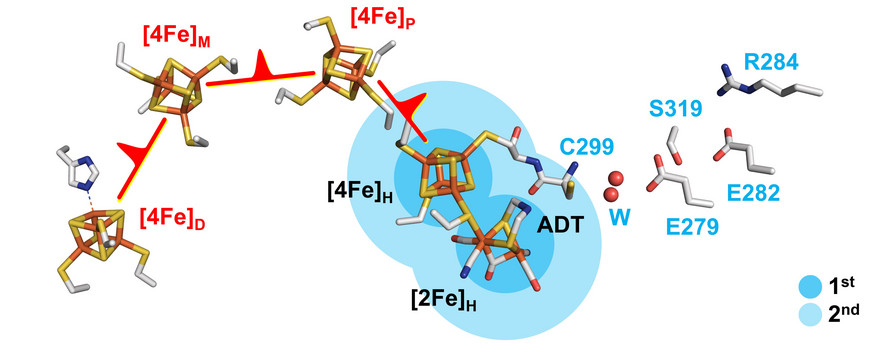
Research focuses on the elucidation of biocatalytic reaction mechanisms with a particular focus on proton-coupled electron transfer (PCET) in metalloenzymes, protein-cofactor interactions, and biological energy conservation such as electron bifurcation and vectorial proton transfer. For many years we have been working on hydrogenases, unique iron-sulfur enzymes that can produce molecular hydrogen (H2). In addition, we are researching enzyme that catalyze N2, O2, and CO2 reduction. Since most of these systems are O2-sensitive, we perform all experiments under strictly anaerobic conditions.
Recent review articles
In situ Infrared Spectroscopy for the Analysis of Gas-processing Metalloenzymes in ACS Catalysis (2021)
Proton Transfer Mechanisms in Bimetallic Hydrogenases in Accounts of Chemical Research (2021)
Second and Outer Coordination Sphere Effects in Low Valent Metalloenzymes in Chemical Reviews (2022)
A Personal Account on 25 Years of Scientific Literature on [FeFe]-hydrogenase in J. Biol. Inorg. Chem. (2023)