Lanthanide spectroscopy
Our research focuses on laser-based optical spectroscopy. Time-resolved absorption and emission spectroscopy are used to investigate various organic and inorganic materials in both solid and liquid phases. The experiments address both fundamental and application-oriented questions, with a current emphasis on the study of lanthanoids and their potential applications.
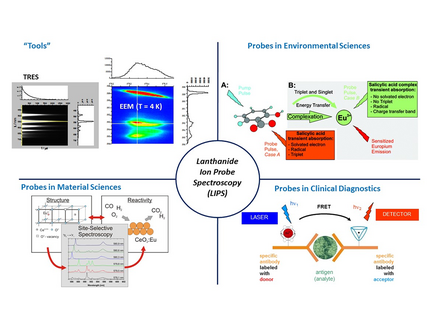
Lanthanoids (Ln(III)) are highly useful luminescent probes and are widely applied in life and environmental sciences as well as in clinical diagnostics. Due to their unique photophysical properties—such as long emission kinetics and narrow-band emission spectra—they are particularly well-suited for luminescence-based analytics and speciation in complex matrices. Time-resolved detection methods allow for efficient suppression of interfering background signals, enabling extremely low detection limits, for example, in sandwich immunoassays.
For investigations in aqueous systems, Eu(III) is primarily used as aluminescent probe, though Dy(III), Sm(III), and Tb(III) are also suitable. The emission parameters of Eu(III) are particularly sensitive to the physicochemical properties of the first (and second) coordination sphere. The emission kinetics of this lanthanoid ion vary depending on the ligands, ranging from a few microseconds to milliseconds, while the spectral position and intensity of different transitions are influenced by the symmetry and type of ligands. The specific electronic characteristics of Eu(III)—such as the combination of magnetic and induced electric transitions, as well as the absence of degeneracy in an electronic transition—significantly determine its properties.
Upconversion luminescence
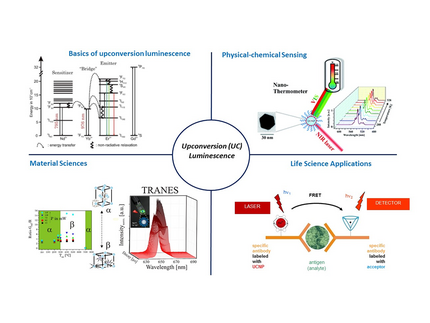
In contrast to "conventional" downconversion—such as fluorescence or phosphorescence in organic dyes, which is widely used for qualitative and quantitative analytics—Upconversion Luminescence (UCL) is based on the absorption of two or more photons.
Although multiphoton processes can also be induced in organic dyes, they typically require complex laser setups. Lanthanides, on the other hand, enable a simpler excitation of such multiphoton processes due to their staggered arrangement of electronic states and exceptionally long deactivation kinetics (in the microsecond range). The electromagnetic radiation required for UCL falls within the near-infrared (NIR) range, which is only weakly absorbed by water or other biological materials. As a result, these rays can penetrate deep into biological matrices—an advantage for UCL-based sensing, which is becoming increasingly important in life and environmental sciences.
In addition to studying the photophysical fundamentals of UCL, applications in biosensing are a key focus. For this purpose, novel crystalline nanoparticles containing lanthanide ions (Ln(III)) are being developed. These nanoparticles (UCNPs) are specifically surface-modified and subsequently characterized as luminescent probes.
Clay and cement sorption
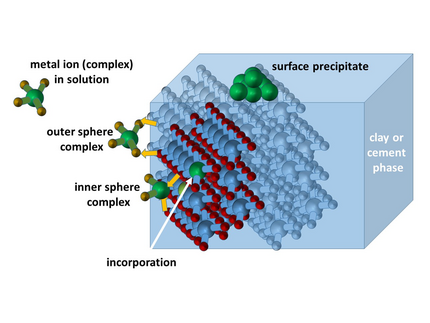
For the safe storage of (heat-generating) radioactive waste, deep geological formations are the preferred option. In addition to salt and granite, clay formations are also considered for this purpose. The overall concept is based on a multi-barrier approach designed to prevent the chemo- and radiotoxic components of the waste (particularly radioactive actinides) from coming into contact with the biosphere. This multi-barrier system integrates technical, geotechnical, and geological components. To ensure the exclusion of radioactive materials over a period of approximately 1,000,000 years, the various components of the multi-barrier system and their interactions with the constituents of radioactive waste must be studied and understood at the molecular level.
For a repository scenario in clay rock, interactions with mineral surfaces (geotechnical and geological barriers) as well as with cement used as a construction material must be characterized. Our research currently focuses on these interactions using lanthanides as natural analogs of actinides (in oxidation state +III), characterized through steady-state and time-resolved luminescence studies. The exceptional luminescence properties of europium are particularly utilized for speciation in these investigations. The materials under study include calcium-silicate-hydrate (CSH) phases and bentonite. CSH phases are key components of cement, while bentonite would serve as a geotechnical barrier.
By employing various spectroscopic parameters (e.g., spectral intensity distribution, luminescence decay time) and experimental techniques such as time-resolved, area-normalized emission spectra (TRANES) and low-temperature luminescence spectroscopy, surface and incorporated species in these materials are analyzed. Special attention is given to system parameters such as pH, ionic strength, and sorption temperature. In addition to binary systems, ternary systems are also examined, where the influence of organic molecules (originating from the stored waste itself or additives in technical materials) on the retention of trivalent actinides (modeled using lanthanides as analogs) is assessed. For speciation, the aforementioned spectroscopic data are evaluated using chemometric data analysis (PARAFAC).
Fluorescent probes
Interactions at cell surfaces are of great fundamental and applied interest in many areas of life sciences, such as the development of novel drugs or therapeutic strategies. Fluorescence methods are distinguished by their exceptional sensitivity and, in principle, can detect individual molecules. In addition to instrumental and technical aspects (such as sensitivity and spatial resolution in imaging applications), (bio)chemical parameters must also be optimized (the availability of highly specific fluorescence probes in particular).
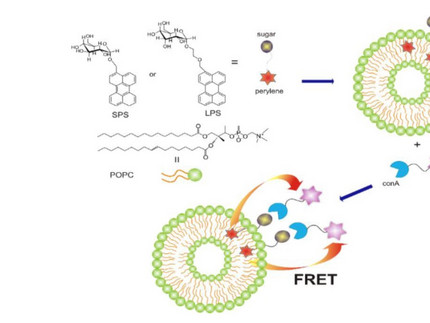
Our research focuses on novel fluorophores and optical probes for biosensing. To systematically vary the complexity of experiments, biomimetic and model systems are often used, such as vesicles as the simplest model for a cell membrane. These novel fluorescence probes are characterized using steady-state and time-resolved optical spectroscopy, providing insights into fundamental photophysical parameters both in solution and in the relevant biological model systems. In addition to excitation and emission spectra as well as fluorescence lifetimes, fluorescence depolarization and transient absorption spectroscopy (TAS) are also employed. This allows for the analysis of spectral intensity distributions, kinetics (down to the sub-picosecond range), and rotational correlation times.
For example, our studies have characterized the interaction of antibodies with vesicles modified by optical probes using fluorescence correlation spectroscopy (FCS) and Förster resonance energy transfer (FRET). In collaboration with the Wessig research group, "molecular rods" have been used as fluorescence probes. Their remarkable rigidity makes them outstanding model systems for FRET probes.
TAS, as a complementary technique to fluorescence spectroscopy, has also been successfully applied to investigate non-radiative deactivation processes, which have previously hindered the speciation analysis of uranyl in natural waters.
Lanthanides as fluorescence probes
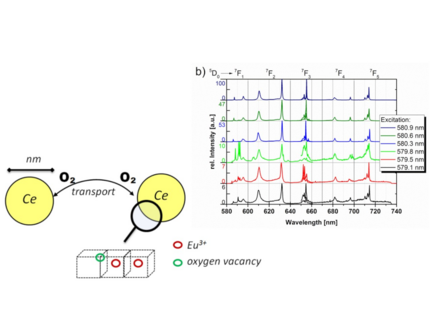
Cerium oxide nanoparticles are known for their exceptional catalytic properties and are widely used in heterogeneous catalysis. A key reason for their high catalytic efficiency is the storage and release of oxygen in/from vacancies within the CeO₂ crystal structure. By introducing dopants, the oxygen mobility can be modified.
In our experiments, we use lanthanides (primarily europium) as "orthogonal" dopants. In addition to altering oxygen mobility, the intrinsic luminescence of lanthanides enables sensitive structural analysis. Europium exhibits outstanding luminescent properties, particularly when emission measurements are performed species-selectively with high spectral and temporal resolution.
Our laser spectroscopic experiments are conducted at T < 10 K to minimize inhomogeneous line broadening. By analyzing spectrally high-resolution excitation-emission matrices (EEM), we obtain complementary structural information on the cerium oxide nanoparticles, alongside XRD and Raman spectroscopy.
Furthermore, another substance class of interest for catalytic applications are inorganic perovskites, which are also studied using the aforementioned techniques. Additionally, electrochemical properties are characterized.
Upconversion nanoparticles (UCNP)
We work with frequency upconverting nanoparticles (upconversion nanoparticles, UCNP) for applications in biosensing and imaging. The excitation of visible luminescence using NIR radiation (frequency upconversion) makes UCNP highly attractive as optical probes, as it allows optimal utilization of the "optical window" in biological samples, enabling deep light penetration into tissues.
Beyond optimizing photophysical properties, key challenges in UCNP development include their transfer into the aqueous phase and surface functionalization—essential steps for successful application in biosensing, clinical diagnostics, or even therapy. Our research addresses the entire spectrum of these tasks, from synthesis and detailed photophysical characterization via steady-state and time-resolved optical spectroscopy to surface functionalization.
As host lattices, we primarily use NaYF₄ and NaScF₄, as their low lattice phonon energies ensure high upconversion efficiency. Within the UCNP, lanthanide ions serve as luminescent emitters, with neodymium and ytterbium typically acting as sensitizers, while erbium and thulium function as activators.